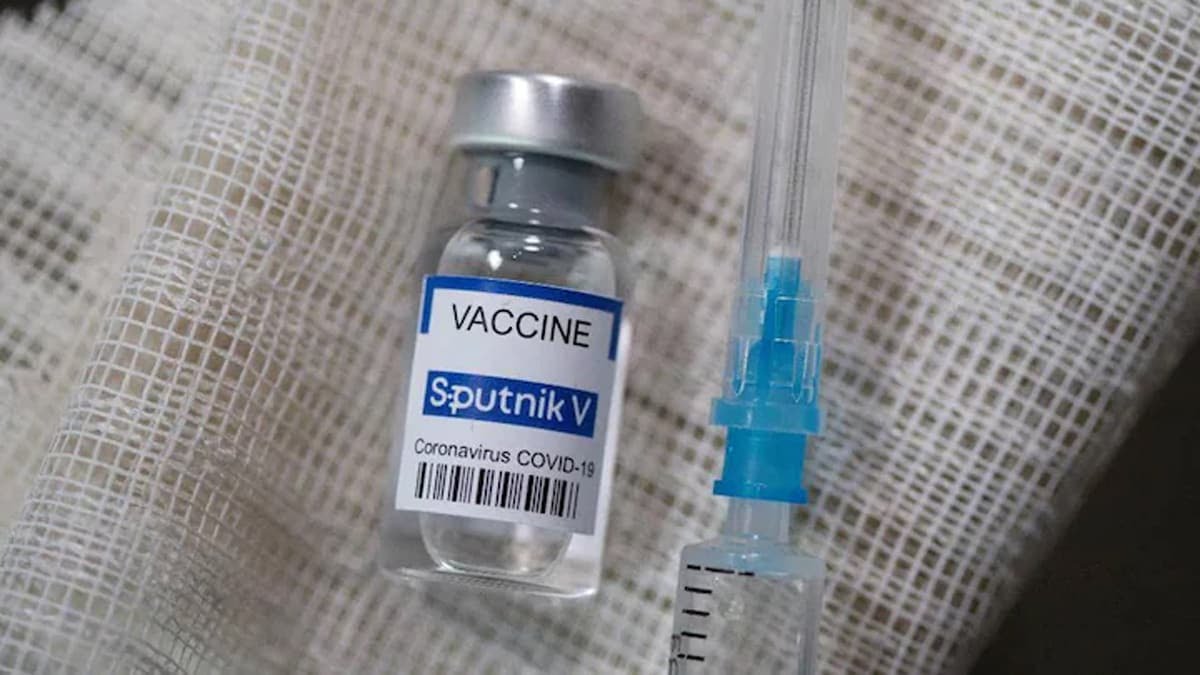
The single-dose COVID-19 vaccine Sputnik Light received approval from the Subject Expert Committee (SEC) of the Drugs Controller General of India (DCGI) today to conduct phase 3 bridging trials in India.
The subject expert committee (SEC) of the Central Drugs Standard Control Organisation, in its 172nd meeting last month, recommended the drugs controller to grant approval to conduct bridging studies in India for the single-dose variant of Sputnik V, after going through updated safety, immunogenicity and efficacy data from phase 3 trials that happened in Russia.
The subject expert committee (SEC) in June asked Dr Reddy’s to submit data from trials in Russia before seeking permission to conduct trials locally.
SEC had in July refused to grant emergency use authorisation (EUA) to Sputnik Light, precluding the need for the conduct of the phase-3 trial of the Russian COVID-19 vaccine in the country.
ALSO READ: Covaxin may get WHO approval by the end of this month – NITI Aayog
Sputnik V vaccine is developed by Russia’s Gamaleya Institute with assistance from Russia’s sovereign wealth fund, Russian Direct Investment Fund (RDIF), that is also marketing the vaccine globally.
RDIF entered a partnership with Dr Reddy’s Labs to market first 250 million doses of the Russian vaccine and also conduct trials in India required for grant of regulatory approvals.
According to Sputnik Light makers, the vaccine has shown an efficacy of 79.4 per cent. If the jab is approved in India, Sputnik Light could be the first single-dose vaccine to be used in the country.
In Russia, the vaccine has been approved for people above 60 years old. The pharma firm has said the vaccine does not cause serious adverse effects.
(For more updates stay tuned with DNP India)